Valence Electrons — Definition & Importance Expii
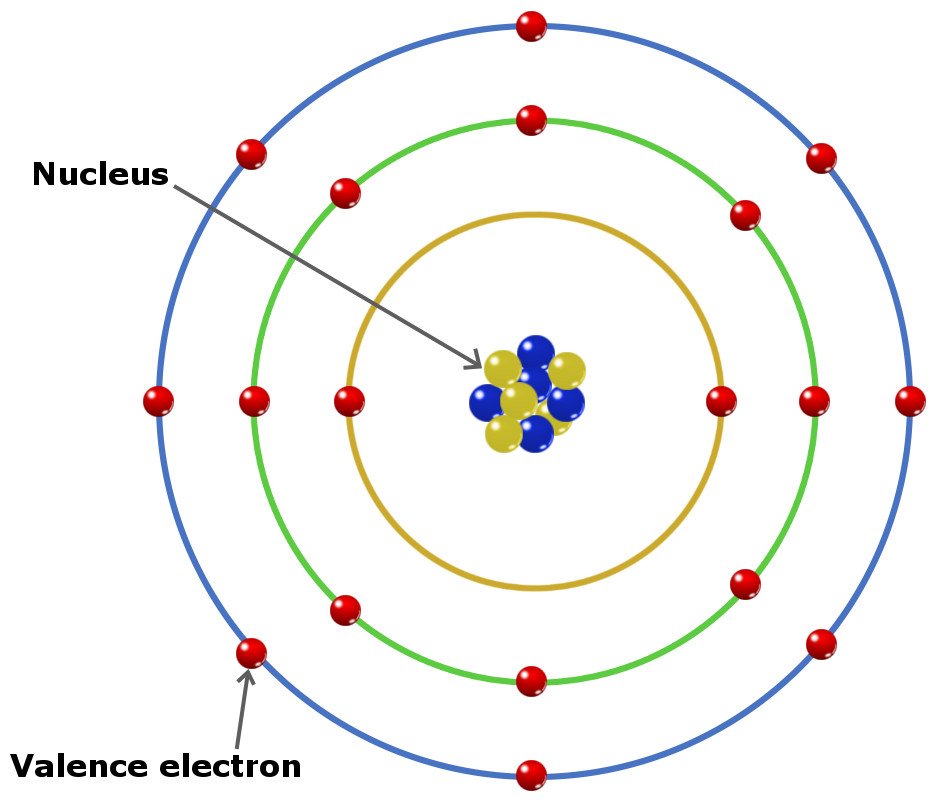
Four covalent bonds.Carbon has four valence electrons and here a valence of four. Each hydrogen atom has one valence electron and is univalent. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both.
Periodic Table Sodium Protons Neutrons Electrons Periodic Table My
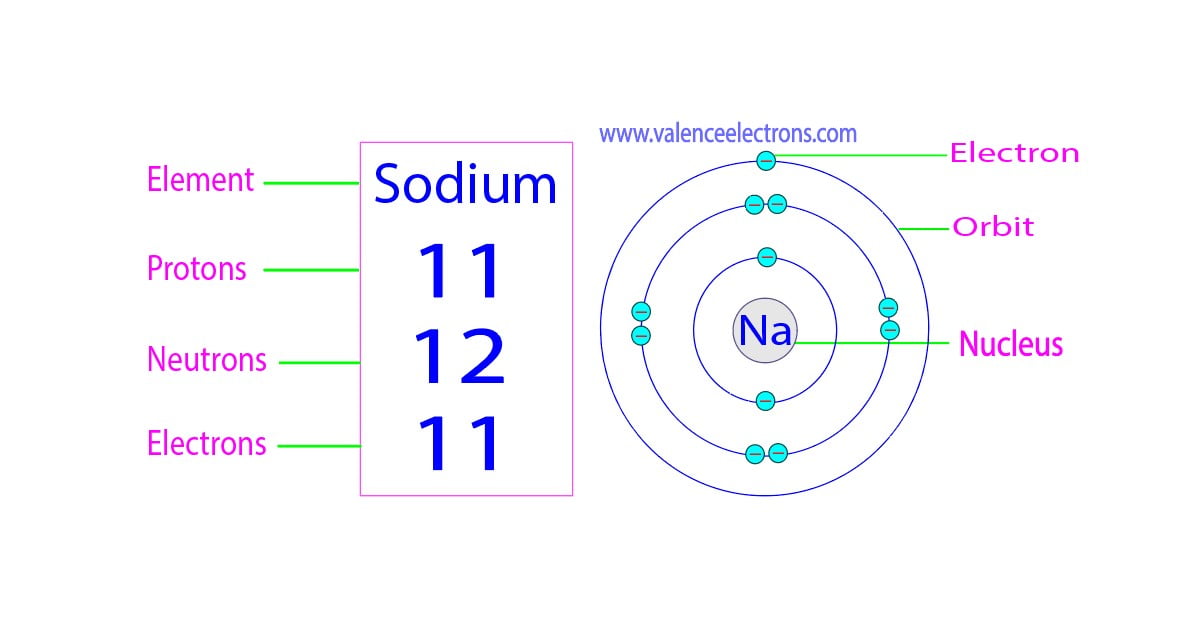
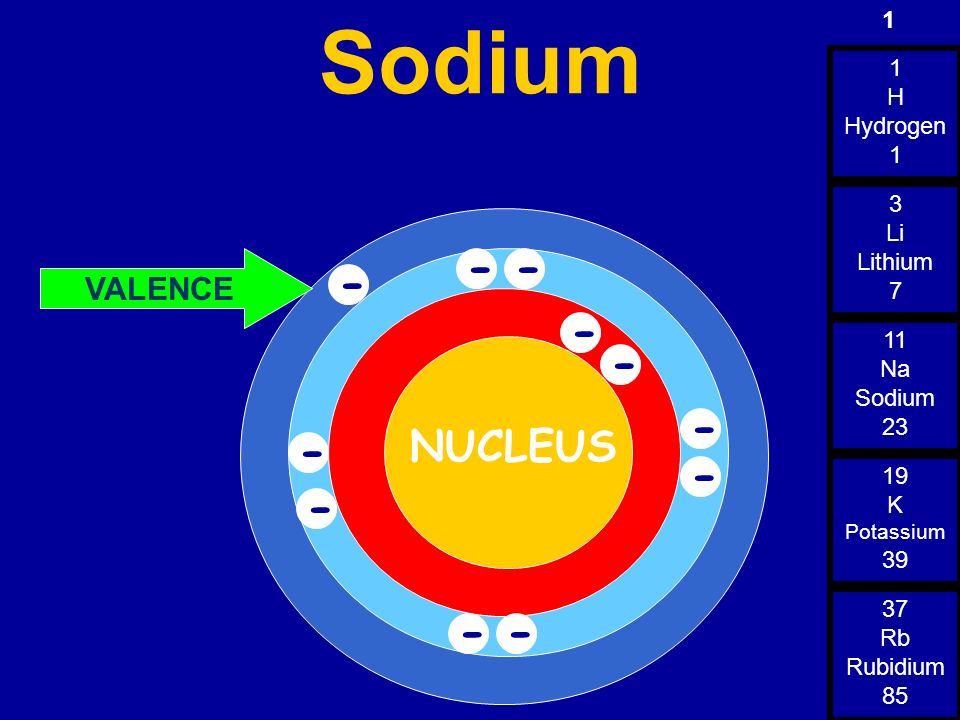
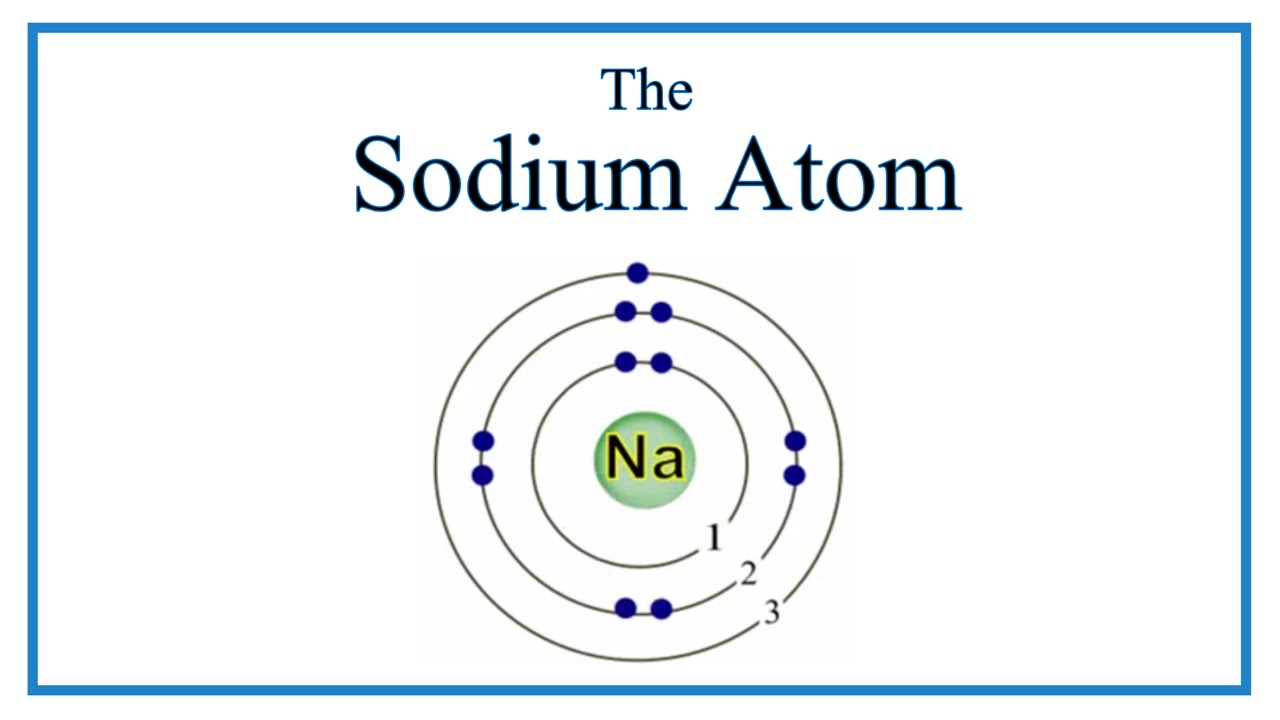
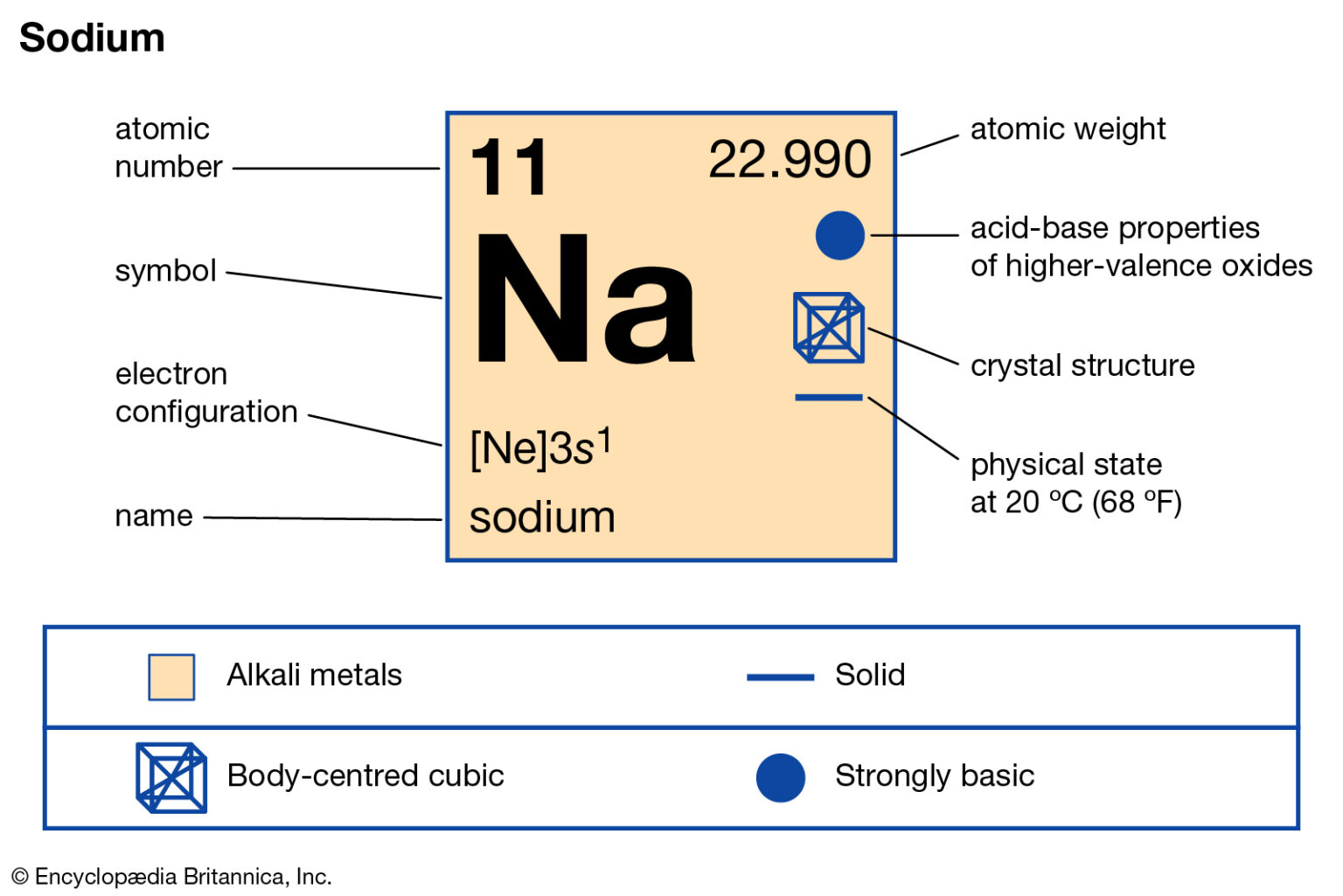
So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. When I want to figure out how many valence electrons sodium has, the number of valence electrons would be equal to the number of electrons in the outermost shell, the outermost energy level. For sodium, sodium has the first energy level, second energy level, and the third energy level.
PPT Classic Chem PowerPoint Presentation, free download ID2464733
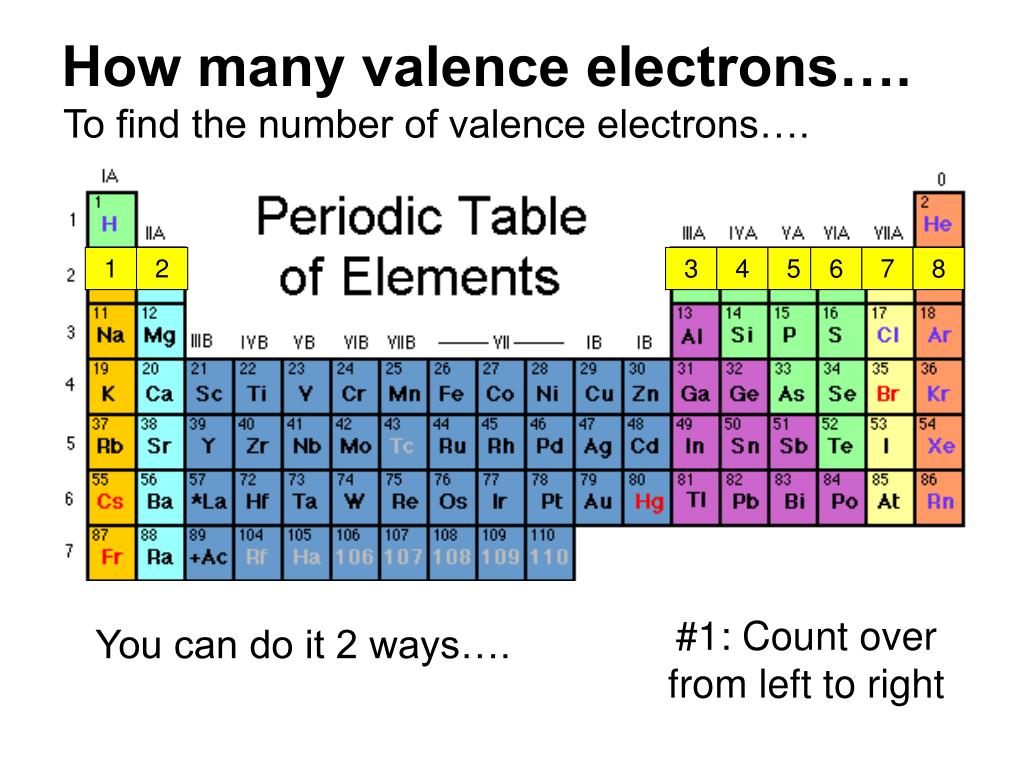
There are two ways to find the number of valence electrons in Sodium (Na). The first is to use the Periodic Table to figure out how many electrons Sodium has.

Sodium Na (Element 11) of Periodic Table NewtonDesk
To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number.
Periodic table of elements with valence electrons kcJuli
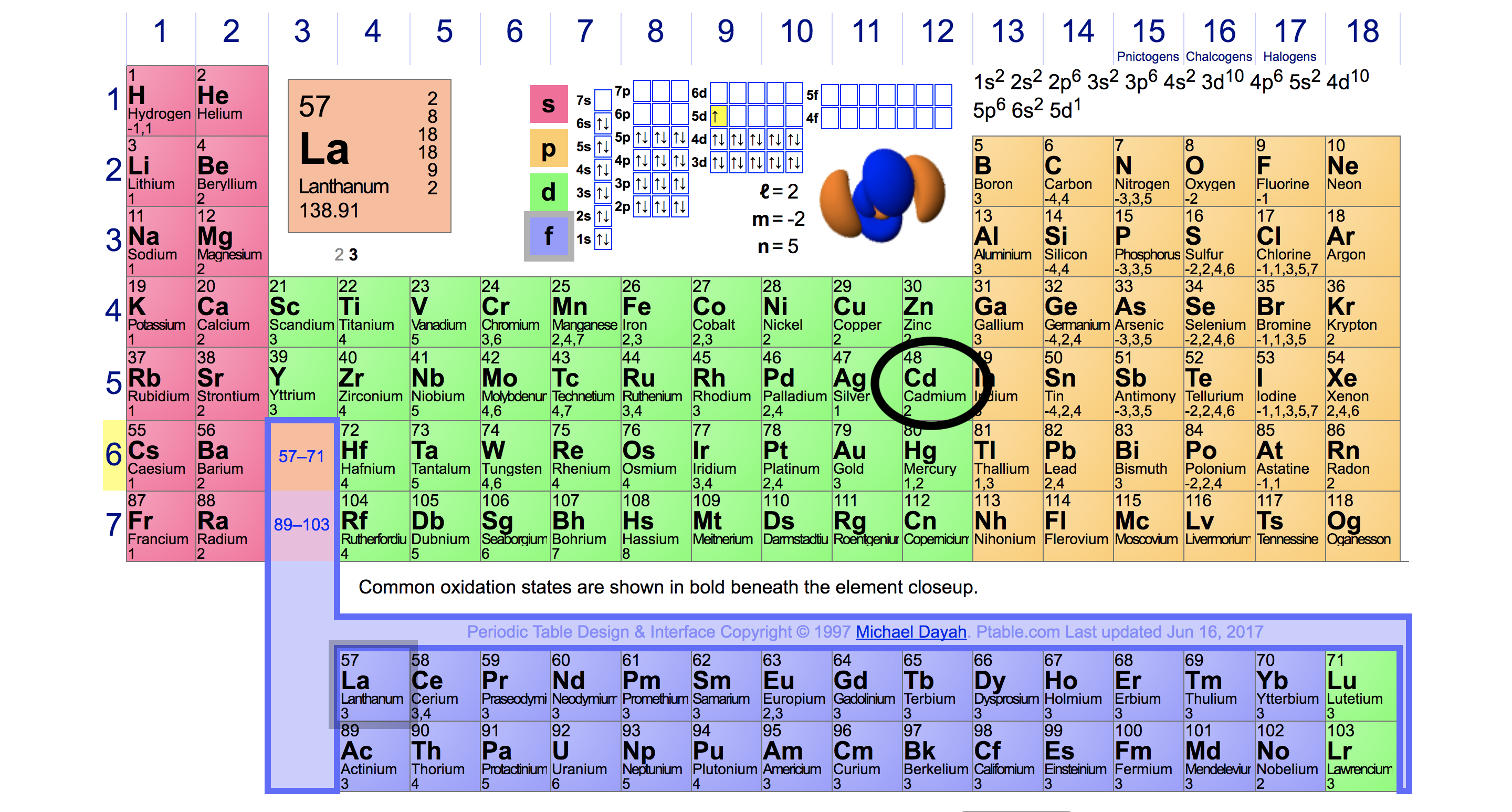
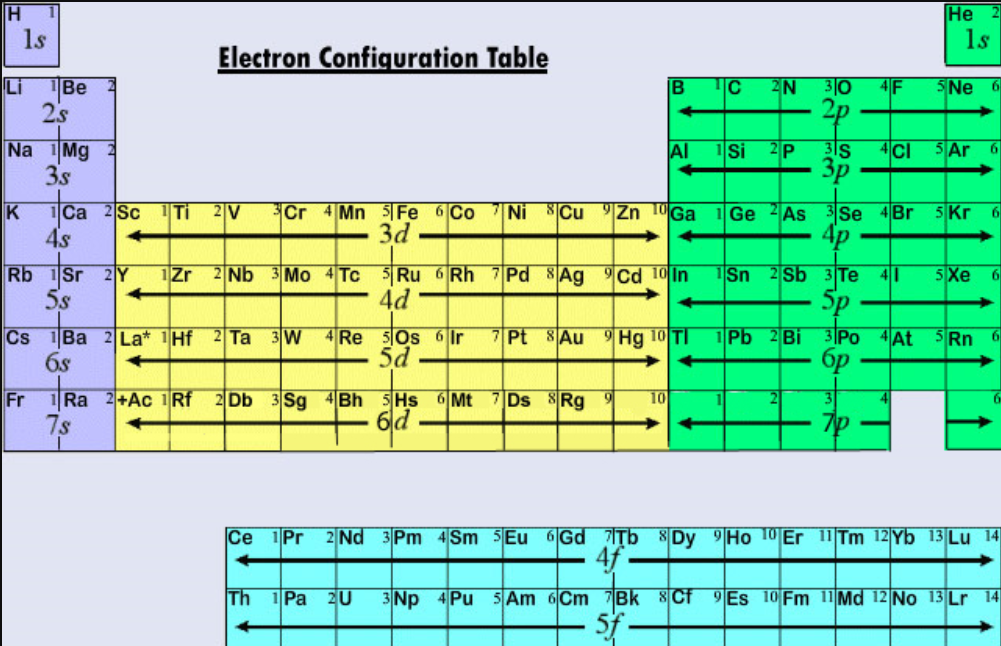
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
How Many Valence Electrons Does Titanium Have rjhinteriordesigndenver
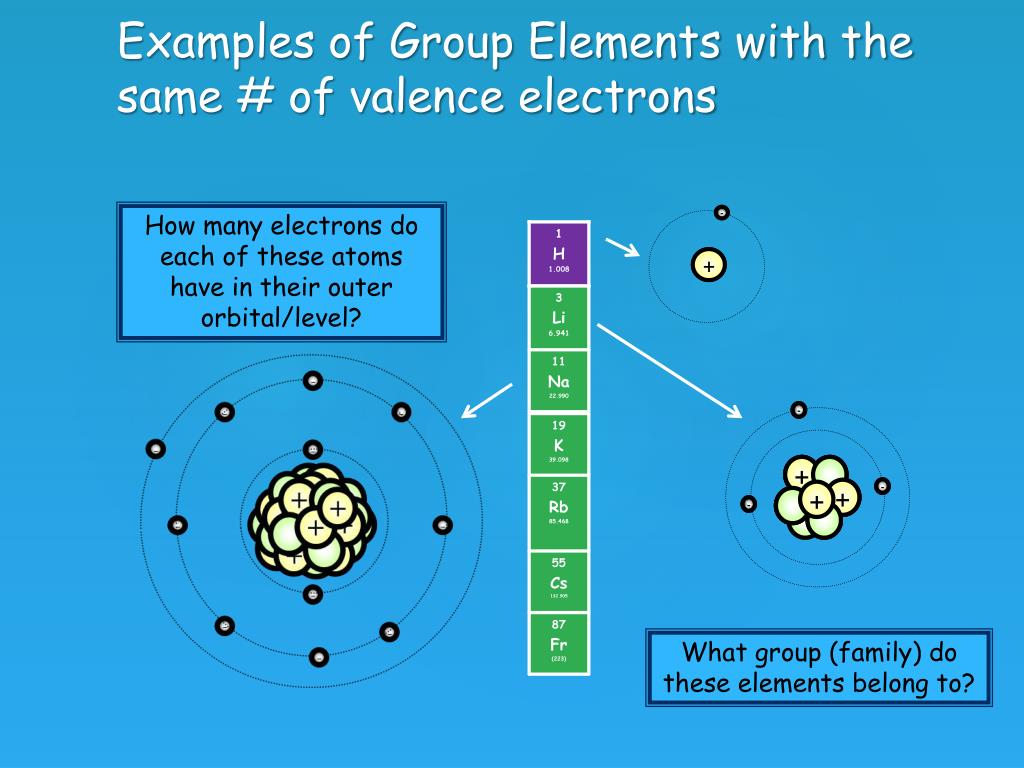
Sodium, with a total of 11 electrons, has only one electron in its third and outermost shell. Because the outermost shell comes into direct contact with other atoms when a chemical reaction takes place, the valence electrons play a big role in determining the chemical reactivity of an element and the elements with which it will react to form.

How to find Valency? What are valence electrons? Teachoo (2023)
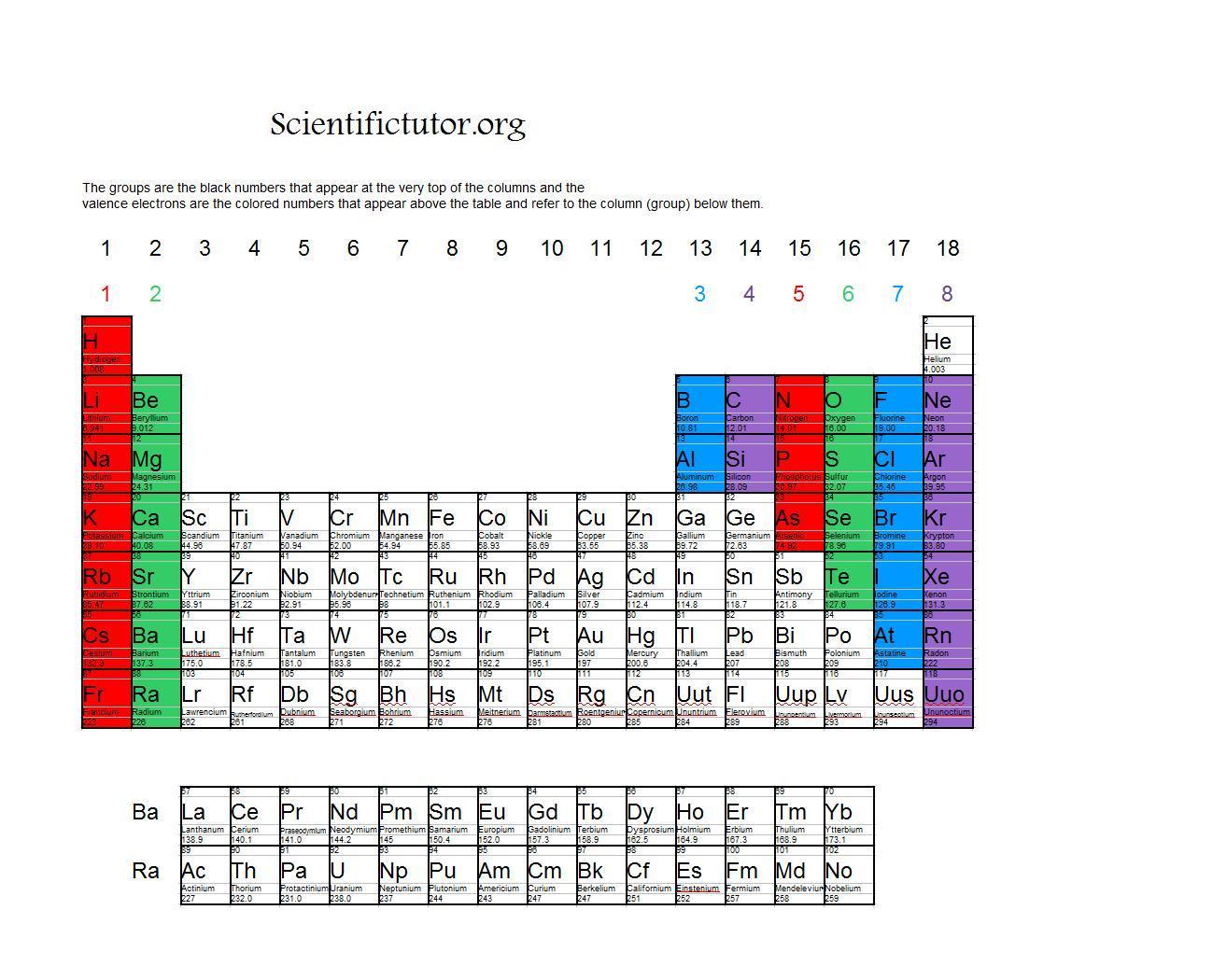
For example, silicon is in Group IVA (Group 14), so each atom would have four valence electrons. Chlorine is in Group VIIA (Group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in Group IIA (Group 2). Helium is the only exception for the main group elements. The first energy level holds a.
Sodium Diagram
For groups 13 to 18, subtract 10 from the group number to get the number of valence electrons, e.g., elements from group 13 will have three valence electrons, from group 14 four valence electrons, and so on. A notable case is group 18, in which the elements (known as noble gases) have 8 valence electrons.
Loadingsalon234
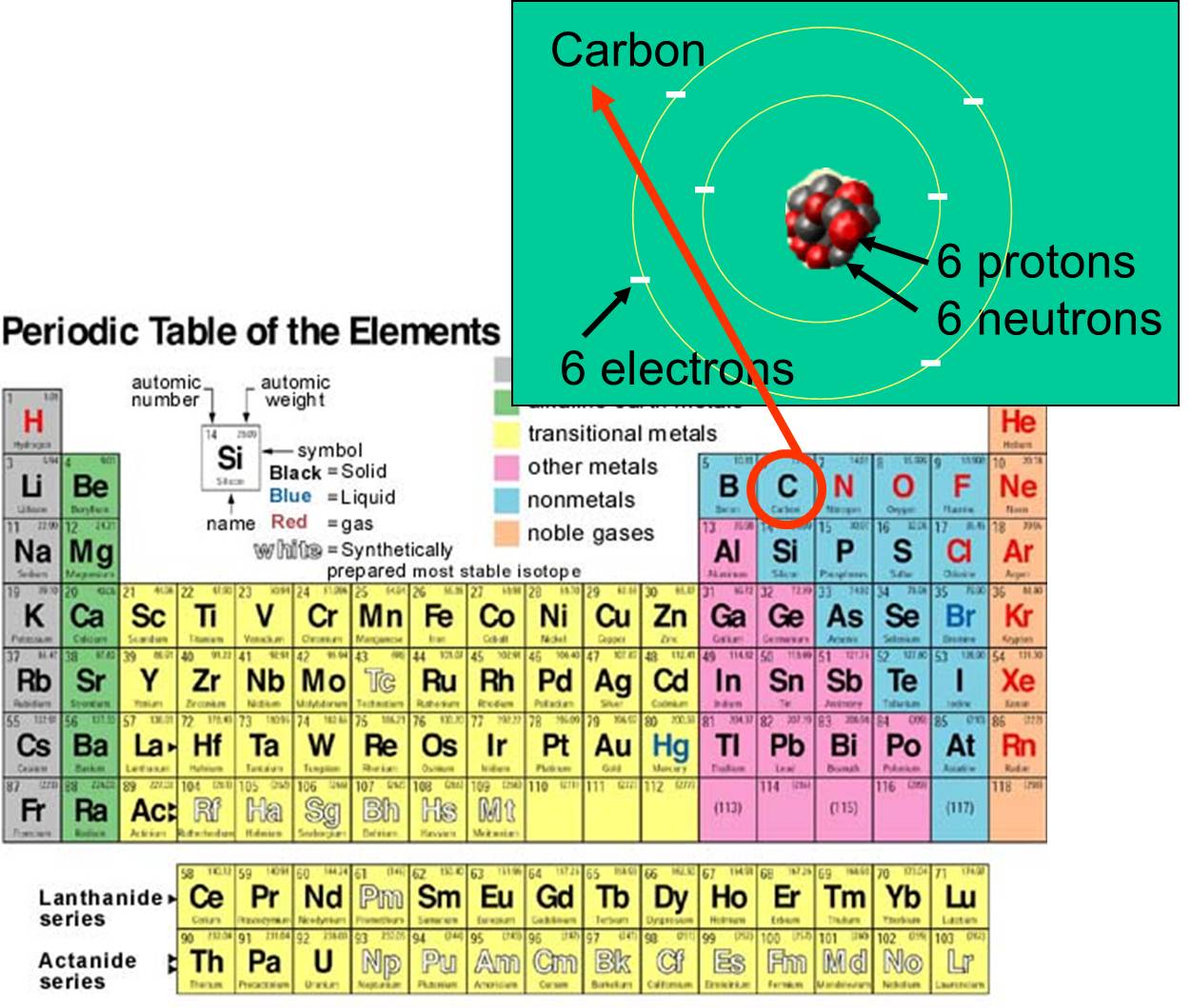
Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1 s 2 2 s 2 2 p 2 —that it has 4 valence electrons (2 s 2 2 p 2 ) and 2 core.
Lead Periodic Table Protons Neutrons And Electrons Matttroy
So, for our example, we would say that sodium has 2 electrons in the 1s orbital plus 2 electrons in the 2s orbital plus 6 electrons in the 2p orbital plus 1 electron in the 3s orbital.. You can use this as a shortcut to determine how many valence electrons an element has — just start from the left side of its period when counting electrons.
Valency of Sodium How many valence electrons does Sodium (Na) have?
In this table, you can see that helium has a full valence shell, with two electrons in its first and only, 1n, shell. Similarly, neon has a complete outer 2n shell containing eight electrons.. So Na has one electron in its outermost orbital. Another example that I'll use is Fluorine (F). Its electronic configuration is 1s2, 2s2, 2p5.

How Many Valence Electrons Does Sodium(Na) Have?
How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. B: 1s 2 2s 2 2p 1 (there are three electrons on the highest occupied energy level n=2)
Electron Dot Diagram Periodic Table
The electron configuration of sodium shows that there is an unpaired electron in the last orbit of sodium. Therefore, the valency of sodium is 1. How many valence electrons does sodium ion(Na +) have? The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation.
PPT Valence Electrons PowerPoint Presentation, free download ID5581383
You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons, respectively. Valence electrons are responsible for the reactivity of an element.

How Many Valence Electrons Does Sodium(Na) Have?
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan.
Sodium Valence Electrons Sodium Valency (Na) with Dot Diagram
Thus, sodium ion (Na+) has eight valence electrons. Na+ valency is not zero like noble gas as their outermost shell has eight electrons. when a sodium atom loses one electron, Na+ ion is produced and that's what valency is. So that Na+ valency is +1 not zero. Chemical Properties Sodium (Na) atom. Atomic number: 11: